*Disseminated on Behalf of Cardiol Therapeutics Inc.
Market Crux Initiates Coverage On Cardiol Therapeutics Inc. (NASDAQ: CRDL) Starting Tomorrow Morning—Monday, August 11, 2025 Here's Why We'll Have All Eyes On (CRDL) Tomorrow Morning…
Analyst Targets Suggest 600% Upside Potential
$2.4B Playbook Offers A Compelling Benchmark For (CRDL)
Phase II Results Show Encouraging Signals In Acute Myocarditis
Advancing Multiple Late-Stage Programs Across Critical Heart Conditions
Backed By A Global Network Of Leading Cardiovascular Specialists
Upcoming Milestones That Could Accelerate Market Recognition
Consider Starting Your Research On (CRDL)
August 10, 2025 Monday's Watchlist | Why (CRDL) Could Be The First Chart You Pull Up Dear Reader, In cardiology, real breakthroughs are rare and the conditions they target often have no approved treatments. That's why a newly released set of clinical trial results is attracting attention from leading research centers across multiple continents. The data points to a therapy that could shift the outlook for a condition linked to sudden loss in otherwise healthy young adults. Cardiol Therapeutics Inc. (NASDAQ: CRDL) just reported topline results from its Phase II ARCHER trial. 
The trial was a global, double-blind, placebo-controlled study evaluating its lead oral drug candidate, CardiolRx™, in patients with acute myocarditis. Acute myocarditis is an inflammatory heart condition and one of the leading causes of sudden cardiac death in people under 35. Long recognized within specialist circles, Cardiol may now be positioned to reach a far wider audience. While ARCHER focuses on myocarditis, Cardiol is also advancing CardiolRx™ in a pivotal Phase III program for recurrent pericarditis—another serious inflammatory heart condition with limited treatment options. This is where the market precedent gets interesting. The $2.4B Blueprint Cardiol Could Follow
In 2021, Kiniksa Pharmaceuticals was valued at roughly $800M. After gaining FDA approval for an injectable biologic to treat recurrent pericarditis, its market cap has grown approximately 200% to more than $2.4B, recently hitting all-time highs. Cardiol Therapeutics Inc. (NASDAQ: CRDL)'s approach to the same condition is fundamentally different. Its therapy is designed to be taken orally, with the goal of being safer, simpler, and more cost-effective than Kiniksa's high-priced, immune-suppressing treatment. And yet, Cardiol's current market cap remains under $100M—a fraction of Kiniksa's valuation—despite several analysts recently setting targets that suggest over 600% upside potential, according to MarketBeat. 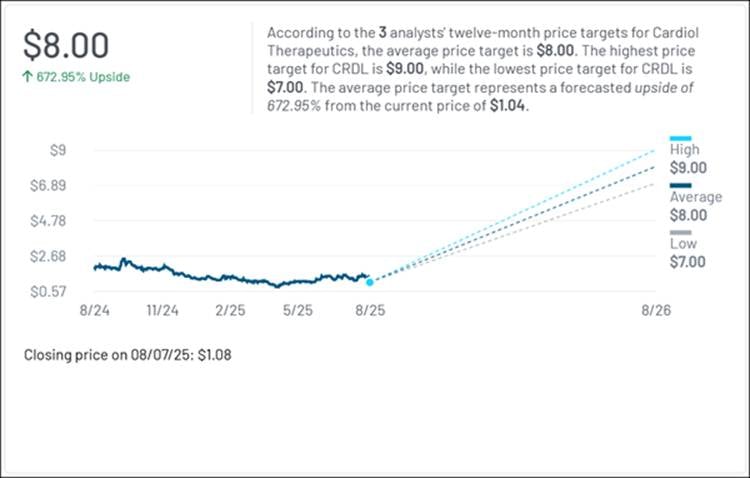
Here's What Just Happened
In the ARCHER trial, patients treated with CardiolRx™ showed: - A notable improvement in left ventricular (LV) extracellular volume (ECV), with a p-value of 0.0538 compared to placebo—trending toward statistical significance.
- A significant reduction in LV mass, a key cardiac MRI metric.
- Improvements across multiple pre-specified CMR endpoints.
- A clean safety and tolerability profile—mirroring outcomes seen in Cardiol's prior MAvERIC Phase II study on pericarditis.
In plain terms: the results show that CardiolRx™ may offer a novel mechanism of action in myocarditis, a condition that currently has no FDA-approved therapies and carries an average hospitalization cost of $110K in the U.S. alone. 
Why This Matters Right Now
- Acute myocarditis is not just rare, it's severely underserved. From viral infections to immune checkpoint inhibitors used in oncology, the causes are wide-ranging. But across the board, treatment remains limited to managing symptoms or complications—there's no standard pharmacological intervention.
- CardiolRx™, a pharmaceutically manufactured cannabidiol, targets the inflammasome pathway, a key driver of inflammation and fibrosis in cardiac tissue. It's these anti-inflammatory and anti-fibrotic properties that could make it relevant as a therapeutic option in recurrent pericarditis, myocarditis, but also in heart failure.
- The ARCHER findings have been submitted for consideration at an upcoming scientific meeting and will be submitted for publication, potentially positioning Cardiol for new attention from academic, regulatory, and industry players alike.
More Than One Shot on Goal
As ARCHER takes the spotlight, Cardiol's flagship MAVERIC trial, a late-stage program with compelling clinical rationale, leads its robust pipeline. MAVERIC Phase III – Recurrent Pericarditis
Cardiol is conducting its pivotal MAVERIC trial in recurrent pericarditis, an inflammatory heart condition with high-cost, and often immunosuppressive treatment options. Phase II MAVERIC-Pilot results (AHA Scientific Sessions 2024) showed: - Rapid pain reduction on the NRS scale.
- Meaningful CRP decline, indicating reduced inflammation.
- Over 70% recurrence-free during the extension period.
Full enrollment in the Phase III trial is expected in the first half of 2026, positioning MAVERIC as the next major milestone. CRD-38 – Heart Failure Program
In parallel, Cardiol is also advancing CRD-38, a subcutaneous formulation of cannabidiol for heart failure, which remains the leading cause of hospitalization in the U.S. and carries a 53% five-year mortality rate. Animal data suggests that CRD-38 could help prevent cardiac remodeling, reduce visceral fat, and modulate inflammation, a potential first-of-its-kind approach in a disease category still dominated by decades-old therapies. See Cardiol's corporate presentation here. Built Around Science, Backed by Experts
What stands out about Cardiol isn't just the programs, it's the depth of collaboration and leadership behind the science. The company has aligned itself with international centers of excellence and enlisted some of the most credible names in cardiovascular research: - Dr. Dennis McNamara (University of Pittsburgh, ARCHER Chair)
- Dr. Leslie T. Cooper Jr. (Mayo Clinic, ARCHER Co-Chair)
- Dr. Allan Klein (Cleveland Clinic, MAVERIC Chair)
- Dr. Massimo Imazio (University of Udine, MAVERIC Co-Chair)
On top of that, its Scientific Advisory Board includes global thought leaders like Dr. Paul Ridker, known for his groundbreaking work on inflammation and heart disease at Harvard and Brigham and Women's Hospital. This is a company executing collaborative, multicenter studies, on the global stage with world-class researchers and clinicians at international centers of excellence. Timing May Be Everything
Cardiol's near-term roadmap is tightly packed with value-driving events: - ARCHER topline results just released (August 6, 2025)
- Full ARCHER data presentation at a major conference (H2 2025)
- MAVERIC Phase III enrollment ongoing
- IND for CRD-38 heart failure program in progress
On the regulatory side, Orphan Drug Designation has already been granted by the FDA for CardiolRx™ for the treatment of pericarditis, and the company is eligible for additional designations for myocarditis in both the U.S. and EU. 
With multiple shots on goal, limited product competition in its lead indications, and a potential best-in-class mechanism of action, Cardiol is positioning itself to challenge the status quo in cardiovascular care. And with fresh Phase II topline data now in hand, analyst coverage heating up, and a pipeline aimed at underserved cardiac conditions, the setup going into this week is hard to ignore. If you're looking for what could dominate tomorrow morning's radar, here's exactly why (CRDL) just jumped to the front of the line. 7 Reasons Why (CRDL) Will Be Topping Our Watchlist Tomorrow Morning —Monday, August 11, 2025
1. Analyst Coverage: Several firms have recently issued targets on (CRDL), with some suggesting levels that imply more than +600% upside potential from its current sub-$100M market cap. 2. Proven Market Blueprint: (CRDL) is targeting recurrent pericarditis, the same condition that helped take Kiniksa from roughly $800M to over $2.4B in market value. 3. Distinct Approach: Unlike Kiniksa's injectable therapy, (CRDL)'s candidate is designed for oral delivery with the goal of being safer, simpler, and more cost-effective. 4. Positive Phase II Topline Data: In the ARCHER trial, (CRDL) showed their drug, CardiolRx™, demonstrated improvements in key cardiac MRI markers, significant reduction in LV mass, and a favorable safety profile. 5. Robust Clinical Pipeline: (CRDL) is advancing three major programs—ARCHER, MAVERIC, and CRD-38—covering myocarditis, recurrent pericarditis, and heart failure. 6. Strong Scientific Network: (CRDL) works with internationally recognized cardiology leaders at top research centers across the U.S., Canada, Europe, Israel, and Brazil. 7. Near-Term Milestones: (CRDL) has several potential catalysts ahead, including a full ARCHER data presentation, MAVERIC Phase III enrollment progress, and CRD-38 IND submission. Final Thoughts: Consider Starting Your Research On (CRDL)
This isn't your typical clinical-stage story. Cardiol Therapeutics Inc. (NASDAQ: CRDL) is operating at the intersection of cardiology, immunology, and innovation—with a science-first approach, respected leadership, and a clear eye on the unmet needs of patients. And now, with ARCHER's topline data released and the MAVERIC Phase III trial underway, the window for early attention may have just opened wider. Several firms have recently issued targets on (CRDL), with some suggesting levels that imply more than 600% upside potential. If you haven't pulled up (CRDL) yet, this might be the moment to do it. Also—watch for my (CRDL) morning update—it could be hitting your inbox bright and early. Sincerely,
Gary Silver
Managing Editor,
Market Crux
| 





Post a Comment
Post a Comment